Air Capture
Air Capture of carbon dioxide (CO2) is the process by which CO2 is taken directly out of the atmosphere after it has already been emitted. Most methods of air capture involve reacting atmospheric CO2 with various chemicals to trap it so that it can be safely sequestered; however, these methods have yet to be implemented commercially, for their economic viability is hotly disputed (Keith, 2009). Because of this, air capture should not be implemented until it has been fully researched to yield a more efficient, and therefore, less costly, method.
Currently, policymakers believe that the best plan of action against increases in CO2 levels in the atmosphere is point source capture, though as CO2 emissions continue to rise, it will be necessary to eventually implement remove CO2 directly from the atmosphere with air capture technology (Keith, 2009).
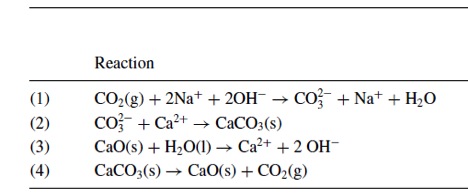
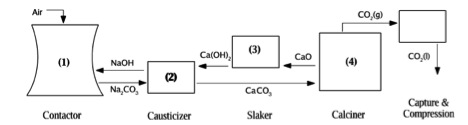
In Air Capture, CO2 is pumped through spray tower that contains mist of sodium hydroxide (NaOH). The NaOH reacts with CO2 to form sodium carbonate (NaCO3), which then react with calcium oxide (CaO) to reform NaOH and produce calcium carbonate (CaCO3). The CaCO3 heated to reform CaO and CO2. This process is called calcining (see Reaction table below).
(Keith, Ha-Duong, & Stolaroff, 2005)
(Source: Keith, D.W., Ha-Duong, M., Stolaroff, J.K. (2005). Climate Strategy With CO2 Capture From the Air. Climatic Change, 74. doi: 0.1007/s10584-005-9026-x)
The plant should be built in places where the wind velocity is less than 10 m/s. It is best to place the air capture as close to the sequestration site as possible, to minimize CO2 transportation costs (Keith, Ha-Duong, & Stolaroff, 2005).
Alkaline Scrubbing (Keith, Ha-Duong, & Stolaroff, 2005):
(Source: Keith, D.W., Ha-Duong, M., Stolaroff, J.K. (2005). Climate Strategy With CO2 Capture From the Air. Climatic Change, 74. doi: 0.1007/s10584-005-9026-x)
Advantages: (Keith, 2009)
- Air capture removes CO2 directly from the atmosphere, so emissions from nonpoint sources are captured as well.
- Air capture is not linked to an energy-producing infrastructure, so air capture structure can be implemented anywhere that space is available. Using air capture near sequestration sites may help decrease the need for CO2 transportation.
Disadvantages: (Keith, Ha-Duong, & Stolaroff, 2005)
- Air capture is more expensive than any other carbon capture technologies (see Cost Estimates, below).
Capacity Estimates: (Keith, Ha-Duong, & Stolaroff, 2005)
- Air capture has the potential to remove the amount of CO2 produced by a 50 W coal-fired power plant (Keith, Ha-Duong, & Stolaroff, 2005).
- Air capture is limited by the flux of air, which is about 1.46 tCO2 /m²-yr.
- Air capture structures need to be placed far enough apart so that the air reaching these structures contains the same concentration of CO2 as the surrounding. Since air capture structures do not take up much space, the land area between structures can be used for other purposes. Therefore, the area of land available will not usually constrain the scale at which air capture is employed.
- If just the area of land each air capture unit is considered, the CO2 neutral flux can be as great as several hundred W/m2.
Costs Estimates: (Keith, Ha-Duong, & Stolaroff, 2005)
Conditions:
Tower diameter: 110m
Air velocity: 2m/s
Capture efficiency: 50%
|
Cost per metric ton of CO2 captured
|
$136/t CO2
|
|
Cost to build single plant that captures 279,000t CO2/yr
|
$12 million
|
|
Operation and Maintenance
|
$400,000/yr
|
|
Total Cost (Cost per metric ton of CO2 captured + building of plant + operation and maintenance)
|
$50,344,000/yr
|
|
Energy cost (Including the energy needed to compress CO2 for storage)
|
4GJ/tC = 1.09 GJ/tCO2
|
Every gallon of gasoline emits 8.8 kg of CO2 (“Emission Facts,” n.d.), an equivalent of .0088 metric tons of CO2. Since it is estimated that each tonne of CO2 removed from the atmosphere would cost $136, gasoline prices would be raised by $1.20.
Readiness:
Air capture technology is not ready to be implemented on a wide scale at the moment nor is it ready commercially. It has been concluded that the best action is to wait at least 25 years (Keith, 2009) before considering implementation for the cost to be more competitive because the cost of implementing air capture is even more expensive than the cost of inaction, which is estimated to be 5% GDP (Stern, 2005), or $3.5 trillion/yr. A total of 2400 Gt CO2 must be removed from the atmosphere over the next 90 years in order to stabilize emissions at 450 ppm of CO2 (Jones, 2009). To then decrease the level of CO2 to 350 ppm, an additional 780 Gt CO2 must be removed from the atmosphere, since every ppm of CO2 contains 7.8 Gt CO2 (Carbon Dioxide Information Analysis Center). This, combined with the CO2 that needs to be captured in order to stabilize emissions, would cost a total of $432 trillion, making the yearly expenditure $4.7 trillion. Because air capture is currently the most expensive carbon capture and sequestration method, it is advised to invest in R&D (research development) right now, giving time for more cost effective and energy efficient methods to emerge. At least ten more years are needed to develop air capture technology that can be implemented at a societal scale (Keith, Ha-Duong, & Stolaroff, 2005).
There would be an incentive to use air capture if the cost of carbon emission permits are higher than that of air capture. David Keith of the University of Calgary has developed an air capture process that he has determined will cost less than $110 per metric ton of CO2 captured (Graham-Rowe, 2007). Right now, the cost of a carbon emission permit is about $10/t CO2. This price is predicted to increase to $50/t CO2, and if the price of air capture can drop below this after more R&D, it is possible that air capture will be an economically feasible option (“Scrubbing the Skies,” 2009).

Alkaline Scrubbing Air Capture tower
Designed by David Keith (2008) Direct Capture of CO2 From Ambient Air. Retrieved from http://www.ucalgary.ca/~keith/AirCapture.html
Other Air Capture Techniques:
Electrochemical Fuel-Cell Concentrator
In an Electrochemical Fuel-Cell Concentrator, the fuel cell provides energy to an anode-cathode pair. Hydrogen is added at the anode, and atmospheric gases (O2, N2, CO2) are added to the cathode. CO2 reacts with the gases to form bicarbonate ions, H CO2͞ , and carbonate ions, CO3 ²͞. The dissociation of water at the anode and the cathode will establish a pH gradient that will drive the HCO2͞ ions and CO3²͞ ions through a membrane. Because of the negative charge of these ions, they will move towards the anode with all the H+ ions. They will then react to form concentrated CO2 gas and water, which are then removed from the system. At the cathode, CO2 depleted air is removed. Under this approach, CO2 is concentrated from the atmosphere at 350 kJ/mol CO2 at 23% efficiency. The major point of improvement would be increasing the current density within the fuel cell unit (Eisaman et. Al., 2009).
Electrodialysis Concentrator
Air is added to a KOH solution, where CO2 will react to form K2 CO2 and KHCO3. This is then sprayed into a series of “stacks”. Each stack consists of the following series: CO2 rich acid solution, anion exchange membrane, basic capture solution, bipolar membrane (separates each stack from the next). K2CO2 and KHCO3 are injected into the basic capture solution, and the bicarbonate ion (dissociated from the original molecule) traverses the anion exchange membrane to the CO2 rich acid solution. Meanwhile, KOH is regenerated in the basic solution. The acid stream that contains bicarbonate ions is depleted of CO2 and returned back to the electrodialysis unit. Under this approach, CO2 could be concentrated from atmospheric concentrations at 62 kJ/mol at 85% efficiency. Points of improvement include higher current densities in the unit, higher pressure concentrators, and increased numbers of stacks (Eisaman et. Al., 2009).

